March 31, 2025: Please refer to the Notice on the Home page.
About the HEAD Study
Study Description & Objectives
The longitudinal mutlicenter head-to-head harmonization of tau PET tracers (HEAD) study aims to standardize and compare the performance of different tau PET tracers in tracking Alzheimer's disease pathophysiology. By achieving this, we seek to enhance the accuracy and comparability of tau imaging results across various research studies and clinical trials. Our objectives include optimizing tau PET processing methods, developing a common scale for tau tracers, and comparing their diagnostic performance and associations with amyloid-beta, neurodegeneration, and cognition. It is our expectation that this standardization will allow datasets to be merged and findings to be generalized, ultimately improving the interpretation of tau PET data in the AD field.
Significance
With millions of people affected by dementia globally, and Alzheimer's disease being the leading cause, understanding and tracking tau pathology is essential. Tau progression correlates closely with clinical symptoms, making tau biomarkers vital for research and clinical settings. The HEAD study addresses the critical need for standardized interpretation of tau imaging to improve the identification and monitoring of AD progression. By providing a harmonized approach to the interpretation of tau PET estimates, the study will provide tools to assist in clinical trial design, patient diagnosis, and treatment planning, with the potential to lead to advancements in patient care.

Study Design
The HEAD study is a multisite longitudinal study of over 700 participants across nine specialized dementia research centers. Participants include young healthy controls, cognitively unimpaired individuals, those with mild cognitive impairment, and AD dementia patients. These individuals undergo a series of assessments, including head-to-head tau PET using the two most widely used tau tracers, Flortaucipir and MK-6240, in addition to a subset of participants additionally undergoing tau PET with PI-2620 and RO948, for a total of 4 tau imaging agents Each participant also undergoes magnetic resonance imaging, amyloid PET, cognitive testing, and blood collection for biomarker assessments. This comprehensive design allows for a thorough comparison of the cross-sectional and longitudinal characteristics of the tau tracers within the same subjects, providing invaluable data on their performance and associations with other biomarkers.
Funding and Support
The HEAD study is funded by the National Institute on Aging award 5R01AG073267. This support from the NIH/NIA enables us to conduct comprehensive and high-quality research, bringing together leading PET experts and resources to address the critical need for standardized tau imaging in Alzheimer's disease.
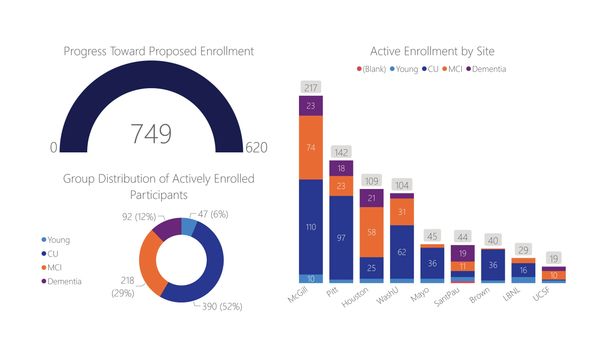
Current Progress in the HEAD Study
The HEAD study has made significant strides in building the cohort and initiating data collection. We have successfully recruited a diverse group of participants across multiple sites, ensuring robust and generalizable results. Data collection is ongoing, with participants currently undergoing baseline assessments and the first follow-up evaluations.
Expected Outcomes
A primary aim of the HEAD study is the development of a universal tau PET scale, which has been named the Uni𝜏 scale. By standardizing tau tracer outcomes, this scale will enhance the interpretability and generalizability of tau PET findings.


